The PJSC Lukoil Oil Company Russian. The teacher will only discuss those questions in which you do not understand the answers supplied in the document.
Saturated fatty acids do not have double bonds in the hydrocarbon chain and unsaturated fatty acids contain at least one double bond.
. Another type of. The IR Spectroscopy of Saturated and Unsaturated Carbons. Most reactions of organic compounds take place at or adjacent to a functional group.
One means of classification depends on the way in which carbon atoms are connected. In this case the concentration is at its fullest making it a stable molecule 87. For this a certain absorbance threshold has been defined which may not be exceeded to put the product on the market as ingredient in medicinal products.
If the chemical does not dissolve is it more or less dense than water. Because of the number and variety of hydrocarbons that can exist some means of classification is necessary. Saturated Vapor Concentration 20C gm3 Calculated 5 Paraffins mm GC 50 Naphthenes mm GC 50 Aromatics mgkg SMS 2728 100 Benzene mgkg GC 3 Sulfur mgkg ISO 20846 05 Flash Point C ASTM D93 67 Lower Explosion Limit in Air vv 06 Upper Explosion Limit in Air vv 60 Auto Ignition Temperature C ASTM E659 255 Electrical Conductivity 20C pSm.
Such compounds are necessarily hydrocarbons made up of chains and. Due to having straight tails saturated fats are dense and solid in nature at room temperature. Based on the molecular formula determine whether each of the following is an alkane alkene or alkyne.
Carbon can form up to four separate bonds with four separate other atoms. However it is also possible for carbon to form multiple bonds with a single atom even another carbon atom. In a previous column 6 we saw that the saturated carbon containing functional groups methyl CH 3 and methylene CH 2 both have C-H stretches below 3000.
All the bonds between carbon atoms are single and the hydrocarbon chain has a straight shape. We will first describe the general case of forming a solution of a molecular species in a liquid solvent and then describe the formation of a solution. Hydrocarbon any of a class of organic chemical compounds composed only of the elements carbon C and hydrogen H.
Kerosene is typically pale yellow or colourless and has a not-unpleasant characteristic odour. Therefore a lot of time will be saved. He proposed that I was controlled by the reciprocal of the fractional water saturation SW to a power n which he named the saturation.
They serve as fuels. We shall discover that we can distinguish all six types of alkenes from each other using IR spectroscopy. Therefore the energy of solution formation the enthalpy of solution equals the sum of the three steps.
To test the solubility of hexane cyclohexene and toluene in water add 1 mL no more of each hydrocarbon to three clean test tubes containing about 5 mL water. You are probably thinking about the point behind the answers at the back of the document. From Hesss law we know that we can add the energies of each step in the cycle to determine the energy of the overall process.
The breaking of bonds requires or absorbs energy. The interactions that determine the solubility of a substance in a liquid depend largely on the chemical nature of the solute such as whether it is ionic or molecular rather than on its physical state solid liquid or gas. This process is endothermic.
Assume that the hydrocarbons are noncyclical and there is no more than one multiple bond a. ˈluːkɔɪl stylized as LUKOIL or ЛУКОЙЛ in Cyrillic script is a Russian multinational energy corporation headquartered in Moscow specializing in the business of extraction production transport and sale of petroleum natural gas and petroleum productsIt was formed in 1991 when three state-run western. The European pharmacopoeia has established a UV photometric procedure to more specifically determine carcinogenic constituents namely polycyclic aromatic hydrocarbons PAHs.
When two carbon atoms in a hydrocarbon are linked together by. D H soln D H 1 D H 2 D H 3. The term saturated refers to the concentration of hydrogen atoms in the carbon chain.
Saturated has a specific definition in terms of carbon-based molecules. It is obtained from petroleum and is used for burning in kerosene lamps and domestic heaters or furnaces as a fuel or fuel component for jet engines and as a solvent for. Kerosene also spelled kerosine also called paraffin or paraffin oil flammable hydrocarbon liquid commonly used as a fuel.
Archie defined a resistivity index I as the ratio of the measured resistivity of the rock Rt to its expected resistivity if completely saturated with water Ro. With each hydrocarbon family alkane alkene alkyne aromatic. The carbon atoms join together to form the framework of the compound and the hydrogen atoms attach to them in many different configurations.
The following day heshe will just check whether you answered them and whether you marked your answers. Alkanes These are referred to as saturated hydrocarbons. Chain aliphatic hydrocarbons are compounds consisting of carbons linked either in a single chain or in a.
A fat molecule made of saturated fatty acids is saturated fat. 12 Some methods of higher. When all the carbons are fully bound to hydrogens the fatty acid is said to be saturated.
Saturated Hydrocarbons Alkanes and Cycloalkanes. It is intended to help. In order to establish a baseline of behavior against which these reactions may be ranked we need to investigate the reactivity of compounds lacking any functional groups.
Shake each mixture for a few seconds and note whether the organic chemical dissolves in water. Hydrocarbons are the principal constituents of petroleum and natural gas. SFAs are hydrocarbon chains with no double bonds.

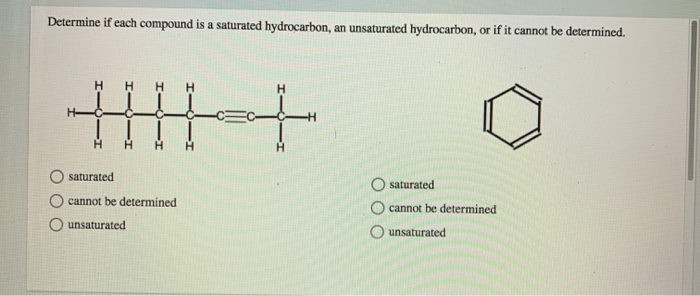
Solved Determine If Each Compound Is A Saturated Chegg Com

0 Comments